SURFACE and STERILIZATION
The Latest Generation, Tissue-Friendly Biphasic Calcium Phosphate Surface
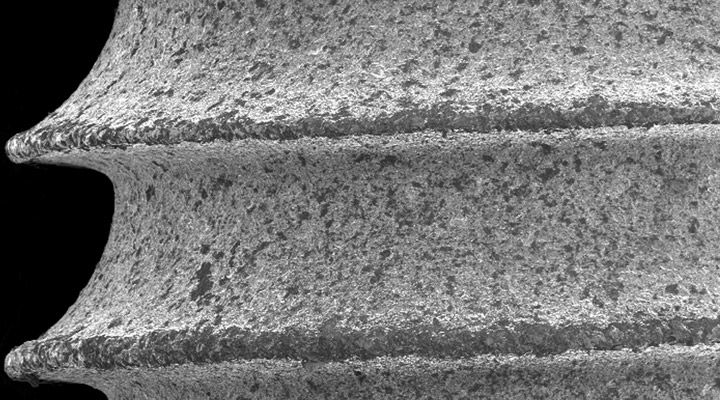
While ImplatechOne is milled from Titanium Grade 4 rods with a production technology that is sensitive to nature and humans, “PURE WATER” is used for cooling purposes and this method eliminates all the negative aspects that oily production may cause on the surface. After the milling process, the implant bodies, which are cleaned by ultrasonic and walnut shell polishing, are made ready for “Calcium Phosphate” surface treatment for optimum bone compatibility and tissue-friendly properties.
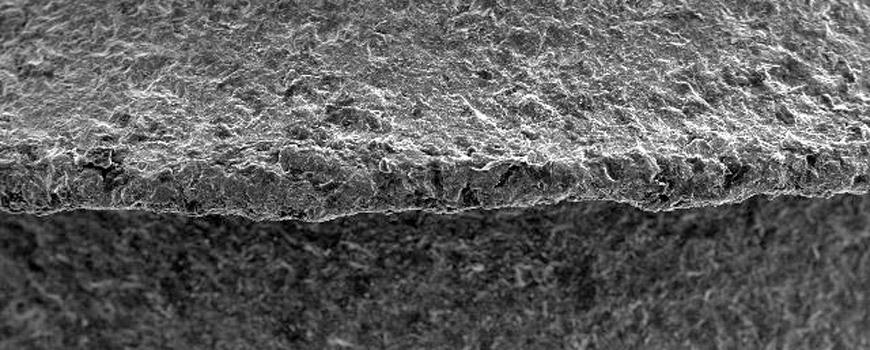
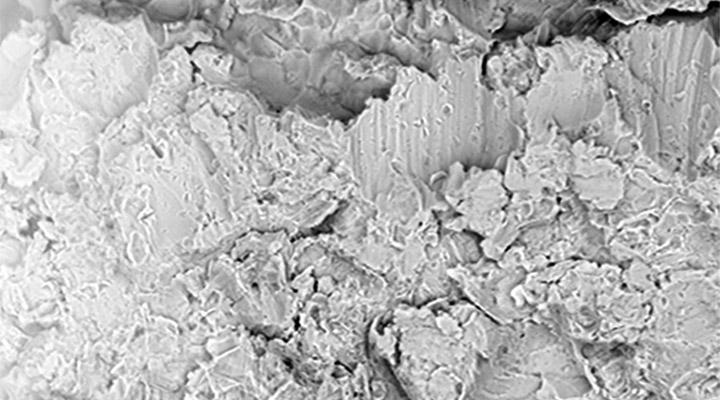
In Calcium Phosphate Surface Morphology, bone-to-implant surface contact ratio is 35% higher than other surfaces. With the Biphasic Calcium Phosphate surface technology from USA, the implant body surface is standardized homogeneously in the 1.4 ~ 1.8-micron range, which is defined as the best surface microporosity range for osseointegration specified in many scientific publications. Implant bodies that come out of the surface treatment process are checked 100% with the latest technology laser and optical systems.
Hydroxylapatite >65%, b-TCP, a-TCP and TTCP phase <35%
Implant bodies with tested surfaces have a shelf life of 5 years with GAMA Sterilization.